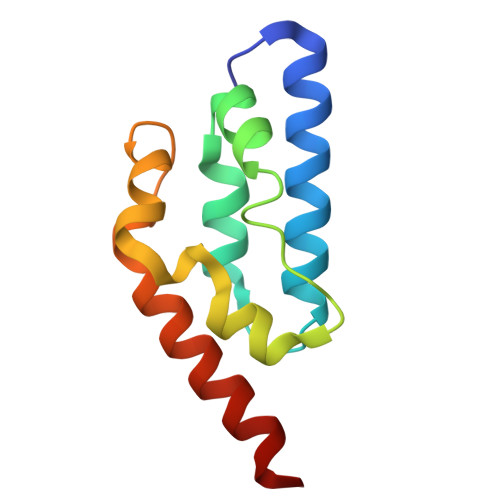
Structural Investigation of the Anti-CRISPR Protein AcrIE7.
Kang, J., Park, C., Lee, G., Koo, J., Oh, H., Kim, E.H., Bae, E., Suh, J.Y.(2025) Proteins 93: 1645-1656
- PubMed: 40318042
- DOI: https://doi.org/10.1002/prot.26832
- Primary Citation of Related Structures:
9LO1, 9LO2 - PubMed Abstract:
The CRISPR-Cas system is an adaptive immune system in prokaryotes that provides protection against bacteriophages. As a countermeasure, bacteriophages have evolved various anti-CRISPR proteins that neutralize CRISPR-Cas immunity. Here, we report the structural and functional investigation of AcrIE7, which inhibits the type I-E CRISPR-Cas system in Pseudomonas aeruginosa. We determined both crystal and solution structures of AcrIE7, which revealed a novel helical fold. In binding assays using various biochemical methods, AcrIE7 did not tightly interact with a single Cas component in the type I-E Cascade complex or the CRISPR adaptation machinery. In contrast, AlphaFold modeling with our experimentally determined AcrIE7 structure predicted that AcrIE7 interacts with Cas3 in the type I-E CRISPR-Cas system in P. aeruginosa. Our findings are consistent with a model where AcrIE7 inhibits Cas3 and also highlight the effectiveness and limitations of AlphaFold modeling.
- Department of Agricultural Biotechnology, Seoul National University, Seoul, Korea.
Organizational Affiliation: