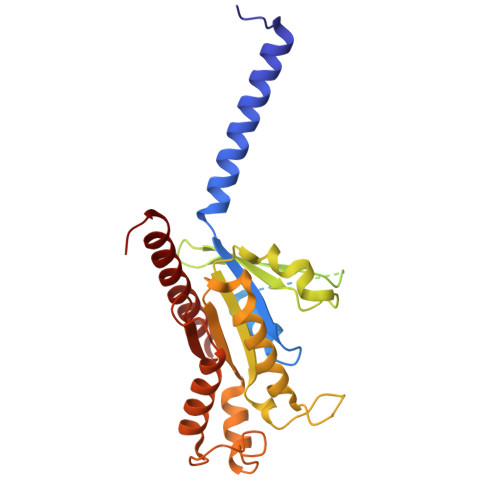
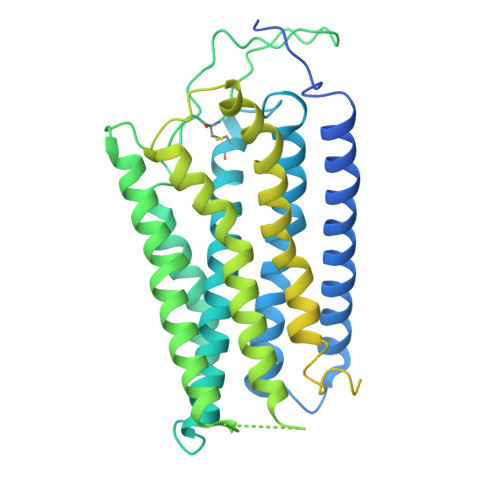
Structural basis of peptide recognition and modulation for neuropeptide FF receptors.
Li, X., Zhang, H., Hu, W., Wu, K., Li, S., Jin, S., Yin, Y., Yuan, Q., Xu, H.E., Pan, B., Jiang, Y.(2025) Cell Rep 44: 116160-116160
- PubMed: 40839429
- DOI: https://doi.org/10.1016/j.celrep.2025.116160
- Primary Citation of Related Structures:
9M0R, 9M1O, 9M2F, 9M54 - PubMed Abstract:
Neuropeptide FF receptors 1 and 2 (NPFFR1 and NPFFR2) are RF-amide peptide receptors that couple to G i/o proteins and regulate pain, opioid tolerance, and metabolism. Despite their physiological significance, their ligand selectivity and activation mechanisms remain unclear. Using cryoelectron microscopy, we resolved four NPFFR1 and NPFFR2 structures bound to NPFF or NPVF, revealing conserved C-terminal RF-amide interactions within the orthosteric pocket and N-terminal variations driving subtype specificity. Structural and mutagenesis analyses identified ECL2 and the receptor N terminus as key determinants of NPVF-NPFFR1 and NPFF-NPFFR2 selectivity. Additionally, the structures elucidate the activation mechanism and uncover distinct G i -coupling features between NPFFR subtypes. These findings provide molecular insights into peptide recognition and receptor activation within the RF-amide family, offering a structural framework for designing selective NPFFR modulators to treat pain, addiction, and metabolic disorders with enhanced specificity and reduced off-target effects.
- School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai 200240, China; Lingang Laboratory, Shanghai, China.
Organizational Affiliation: