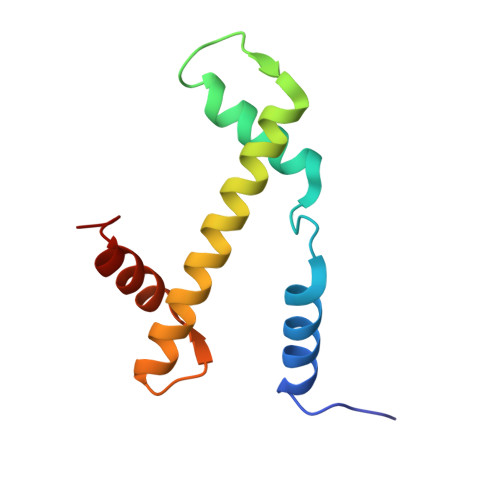
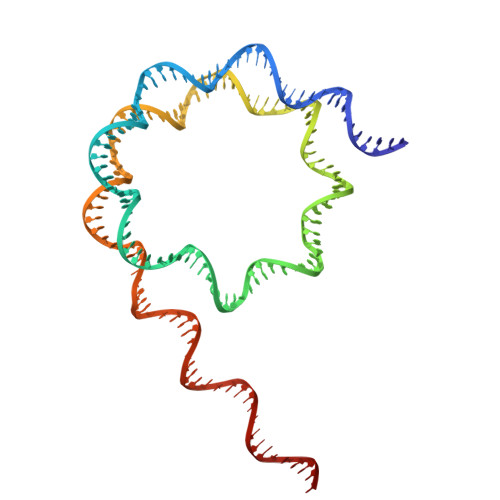
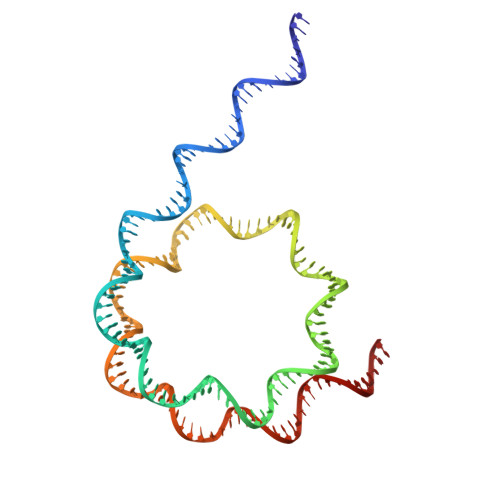
A competitive regulatory mechanism of the Chd1 remodeler is integral to distorting nucleosomal DNA.
Nodelman, I.M., Folkwein, H.J., Glime, W.S., Armache, J.P., Bowman, G.D.(2025) Nat Struct Mol Biol 32: 1445-1455
- PubMed: 40437259
- DOI: https://doi.org/10.1038/s41594-025-01556-y
- Primary Citation of Related Structures:
9N6H, 9N6I, 9N6K - PubMed Abstract:
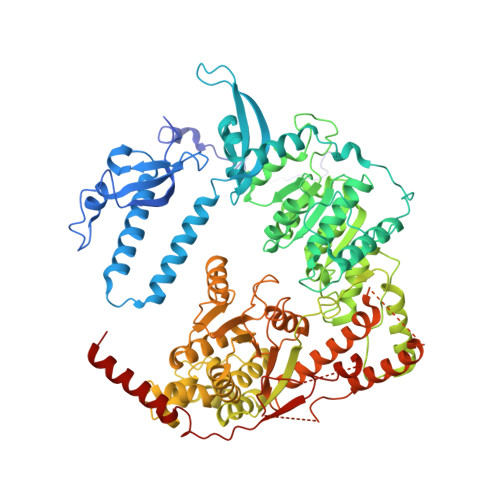
The Chd1 chromatin remodeler repositions nucleosomes into evenly spaced arrays, a characteristic of most eukaryotic genes. Here we show that the yeast Chd1 remodeler requires two activating segments to distort nucleosomal DNA into an A-form-like conformation, a critical first step in nucleosome sliding. As shown by cryo-electron microscopy, these two activating segments together pack against the ATPase motor, where they are poised to stabilize the central ATPase cleft. These activating elements contact the ATPase at locations that are incompatible with binding of NegC, an autoinhibitory segment located between the two activators. NegC inhibits sliding by antagonizing the activators through steric competition and constraining activator placement, giving rise to directional nucleosome sliding. Given that activator reinforcement of the ATPase cleft is needed for DNA distortion, this first step in remodeling appears to provide a natural checkpoint for regulation of chromatin remodeler activity.
- Thomas C. Jenkins Department of Biophysics, Johns Hopkins University, Baltimore, MD, USA.
Organizational Affiliation: