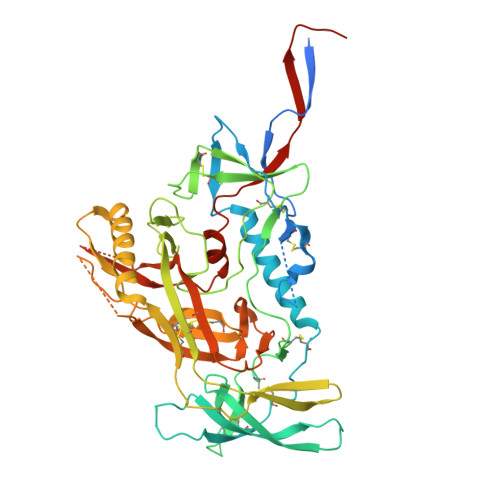
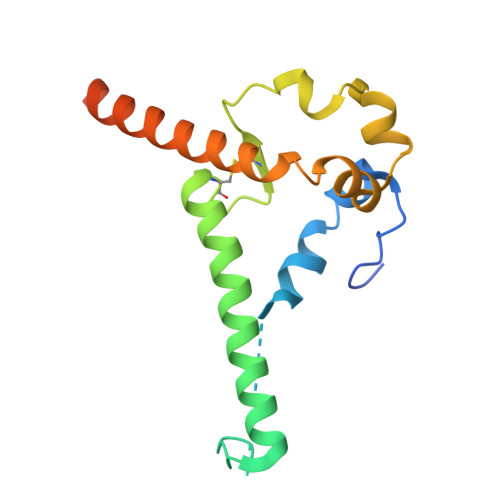
A modification to heptad repeat 1 of gp41 improves yield and/or quality of soluble pre-fusion HIV-1 envelope glycoprotein trimers.
Chaturbhuj, D.N., Sliepen, K., Cupo, A., Steinberg, B., Kazimierczyk, S., Munawar, T., Kramer, K., Yasmeen, A., Andrade, T.G., Lee, W.-H., van der Maas, L., Gibson, G., Feliciano, O., Del Moral Sanchez, I., Schermer, E., Bronson, R., Benner, A., Prabhakaran, M., Mason, R., Klasse, P.J., Ward, A.B., Ozorowski, G., Sanders, R.W., Moore, J.P.(2025) J Virol 99: e0091325-e0091325
- PubMed: 40862550
- DOI: https://doi.org/10.1128/jvi.00913-25
- Primary Citation of Related Structures:
9OGT, 9OGU - PubMed Abstract:
Native-like HIV-1 envelope glycoprotein (Env) trimers, exemplified by the SOSIP design, are widely used as immunogens, analytical antigens, and for structural studies. These vaccine research and development programs require trimers that are based on multiple HIV-1 genotypes. While a wide range of protein engineering strategies can produce SOSIP trimers from most Env gene sequences, there are still examples of trimers that are expressed only at impractically low yields or that are unstable. Accordingly, additional protein modifications aimed at overcoming such limitations need to be evaluated. Here, we describe a new heptad repeat 1 modification of gp41, known as dPG, that helps to further stabilize the gp41 component of prototypic and germline-targeting SOSIP trimers in the pre-fusion state and thereby increases post-purification yields substantially. The dPG modification involves a deletion (d) at the highly conserved 566 position that disrupts the heptad repeat and introduces proline (P) and glycine (G) substitutions at positions 567 and 568, respectively. We show that the dPG strategy reinforces previously described stabilization changes in existing SOSIP trimers and can rescue otherwise problematic trimer constructs. The latter includes trimers used to target or analyze human germline antibodies and others derived from the global HIV-1 neutralization panel. In summary, the dPG modification strategy can increase the yield and/or quality of Env trimers that are otherwise difficult to produce. Stabilized, soluble, pre-fusion SOSIP trimers are widely used in HIV-1 Env vaccine research. Protein engineering techniques have identified multiple ways to stabilize SOSIP trimers from a range of genotypes. However, some SOSIP trimers remain difficult to express at adequate yields and/or purity, so there is a need for additional modifications. Here, we identified a sequence change, designated dPG, to the gp41 subunit that increases the yield and/or quality of various otherwise problematic SOSIP trimers without compromising their antigenicity or structure. This new modification may have general value for HIV-1 vaccine research and development.
- Department of Microbiology and Immunology, Weill Cornell Medicine, New York, New York, USA.
Organizational Affiliation: