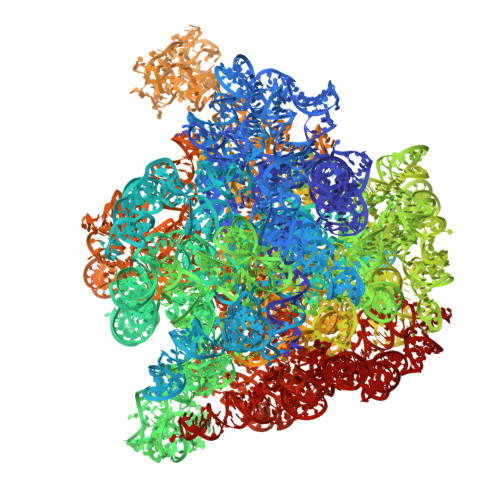
Cryo-EM Study and In Vivo Chemical Mapping of the Methanosarcina acetivorans Ribosome and Its Dimerization via a Repurposed Enzyme and Translation Factor.
Fordjour, G.N.R., Ghosh, A., Ferry, J.G., Armache, J.P., Bevilacqua, P.C., Murakami, K.S.(2025) J Biological Chem : 110686-110686
- PubMed: 40914243
- DOI: https://doi.org/10.1016/j.jbc.2025.110686
- Primary Citation of Related Structures:
9NRI, 9NTA, 9O17, 9OU7 - PubMed Abstract:
Despite the overall conservation of ribosomes across all domains of life, differences in their 3D architecture, rRNA sequences, ribosomal protein composition, and translation factor requirements reflect lineage-specific adaptations to environmental niches. In the domain Archaea, structural studies have primarily focused on non-methanogenic thermophiles and halophiles, leaving it unclear whether these represent the broader archaeal domain. Here, we report the cryo-electron microscopy (cryo-EM) structure of the ribosome from Methanosarcina acetivorans, a previously unreported high-resolution structure from a model mesophilic methanogenic archaeon. Compared to ribosomes from extremophiles, the M. acetivorans ribosome has a simplified architecture, lacking paralogous duplications and containing a reduced complement of ribosomal proteins. Structures of the large subunit (50S) from cells grown with either methanol or acetate show conserved rRNA folding and protein composition. High-resolution structures of the 50S subunit from the two growth substrates enabled us to investigate structural properties that may influence in vivo dimethyl sulfate (DMS) reactivity, an orthogonal chemical approach used to probe RNA structure. We observed good agreement between in vivo DMS reactivity and ribosome structure. Finally, we identify a previously uncharacterized ribosome dimerization mode involving only 50S subunits and mediated by a heterotetrameric complex of PurH and aEF2-proteins with alternative metabolic and translational roles. This macromolecular assembly, which we term the Methanogen Ribosome Dimerization Factor (MRDF), likely mediates ribosome hibernation, revealing an alternative regulatory mechanism in translation.
- Department of Biochemistry and Molecular Biology, Penn State University, University Park, PA, 16802; Center for Structural Biology, Penn State University, University Park, PA 16802; Center for RNA Molecular Biology, Penn State University, University Park, PA 16802; Center for Eukaryotic Gene Regulation, Penn State University, University Park, PA 16802; Molecular Machines Mechanism and Structure Predoctoral Training Program, Penn State University, University Park, PA 16802.
Organizational Affiliation: