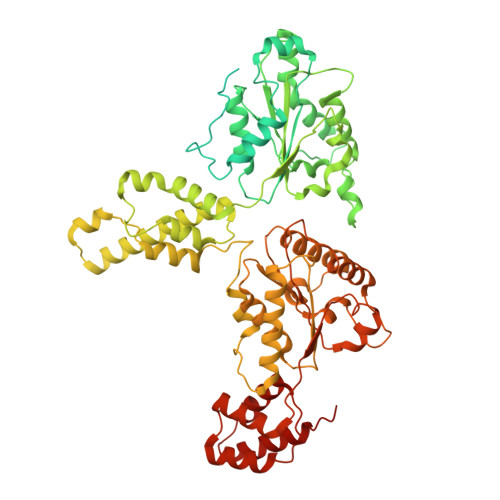
Structural remodeling of target-SNARE protein complexes by NSF enables synaptic transmission.
White, K.I., Khan, Y.A., Qiu, K., Balaji, A., Couoh-Cardel, S., Esquivies, L., Pfuetzner, R.A., Diao, J., Brunger, A.T.(2025) Nat Commun 16: 8371-8371
- PubMed: 40993127
- DOI: https://doi.org/10.1038/s41467-025-62764-0
- Primary Citation of Related Structures:
9OJR, 9OJU, 9OJZ, 9OK3, 9OK5, 9OKC, 9OLJ, 9OLO, 9OM6, 9OMQ, 9PAF, 9PAG, 9PB9, 9PBA, 9PBF, 9PBV, 9PC3, 9PCX, 9PCZ, 9PD1, 9PD8, 9PDB, 9PDD, 9PF2, 9PFC, 9PFF, 9PFG - PubMed Abstract:
Synaptic vesicles containing neurotransmitters fuse with the plasma membrane upon the arrival of an action potential at the active zone. Multiple proteins organize trans-SNARE complex assembly and priming, leading to fusion. One target membrane SNARE, syntaxin, forms nanodomains at the active zone, and another, SNAP-25, enters non-fusogenic complexes with it. Here, we reveal mechanistic details of AAA+ protein NSF (N-ethylmaleimide sensitive factor) and SNAP (soluble NSF attachment protein) action before fusion. We show that syntaxin clusters are conserved, that NSF colocalizes with them, and characterize SNARE populations that may exist within or near them using cryo-EM. Supercomplexes of NSF, α-SNAP, and either a syntaxin tetramer or one of two binary complexes of syntaxin-SNAP-25 reveal atomic details of SNARE processing and show how sequential ATP hydrolysis drives disassembly. These results suggest a functional role for syntaxin clusters as reservoirs and a corresponding role for NSF in syntaxin liberation and SNARE protein quality control preceding fusion.
- Department of Molecular and Cellular Physiology, Stanford University, Stanford, CA, USA. kiwhite@stanford.edu.
Organizational Affiliation: