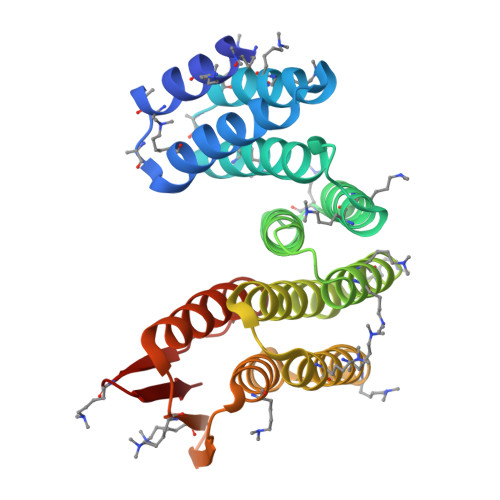
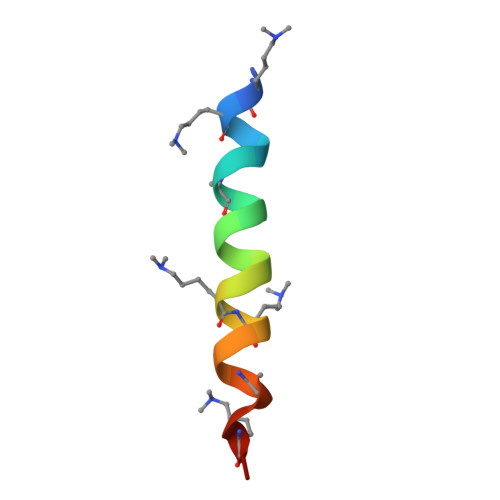
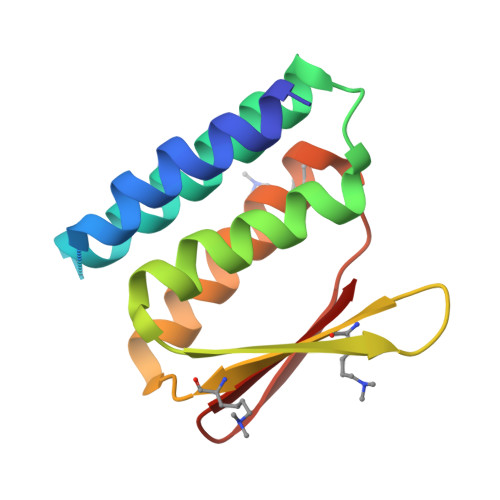
Design of facilitated dissociation enables timing of cytokine signalling.
Broerman, A.J., Pollmann, C., Zhao, Y., Lichtenstein, M.A., Jackson, M.D., Tessmer, M.H., Ryu, W.H., Ogishi, M., Abedi, M.H., Sahtoe, D.D., Allen, A., Kang, A., De La Cruz, J., Brackenbrough, E., Sankaran, B., Bera, A.K., Zuckerman, D.M., Stoll, S., Garcia, K.C., Praetorius, F., Piehler, J., Baker, D.(2025) Nature
- PubMed: 40993395
- DOI: https://doi.org/10.1038/s41586-025-09549-z
- Primary Citation of Related Structures:
9DCX, 9DCY, 9DCZ, 9DD0, 9DD1, 9DD2, 9DD3, 9DD4, 9DD5, 9OLQ - PubMed Abstract:
Protein design has focused on the design of ground states, ensuring that they are sufficiently low energy to be highly populated 1 . Designing the kinetics and dynamics of a system requires, in addition, the design of excited states that are traversed in transitions from one low-lying state to another 2,3 . This is a challenging task because such states must be sufficiently strained to be poorly populated, but not so strained that they are not populated at all, and because protein design methods have focused on generating near-ideal structures 4-7 . Here we describe a general approach for designing systems that use an induced-fit power stroke 8 to generate a structurally frustrated 9 and strained excited state, allosterically driving protein complex dissociation. X-ray crystallography, double electron-electron resonance spectroscopy and kinetic binding measurements show that incorporating excited states enables the design of effector-induced increases in dissociation rates as high as 5,700-fold. We highlight the power of this approach by designing rapid biosensors, kinetically controlled circuits and cytokine mimics that can be dissociated from their receptors within seconds, enabling dissection of the temporal dynamics of interleukin-2 signalling.
- Institute for Protein Design, University of Washington, Seattle, WA, USA. broerman@uw.edu.
Organizational Affiliation: