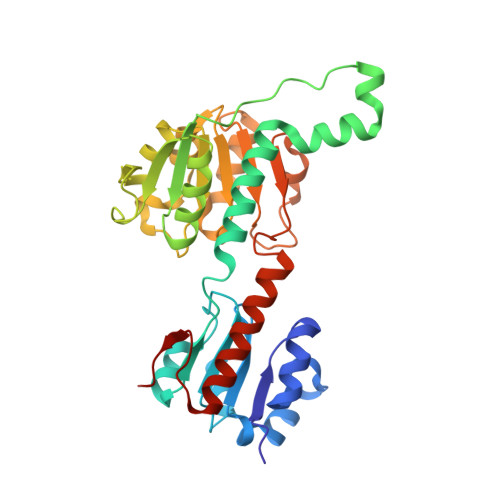
Crystallization and structural analysis of 2-hydroxyacid dehydrogenase from Ketogulonicigenium vulgare.
Han, X., Xiong, X., Hu, X., Li, M., Zhang, W., Liu, X.(2014) Biotechnol Lett 36: 295-300
- PubMed: 24068509
- DOI: https://doi.org/10.1007/s10529-013-1354-8
- Primary Citation of Related Structures:
4LSW - PubMed Abstract:
L-2-Hydroxyacid dehydrogenase (HDH) from Ketogulonicigenium vulgare Y25 was cloned and overexpressed in Escherichia coli. The protein was purified and crystallized by the sitting-drop vapour-diffusion method with polyethylene glycol 3350 as precipitant. The crystal structure of HDH was determined at 1.64 Å resolution using the molecular replacement method with the crystal structure of hydroxyl (phenyl) pyruvate reductase from Coleus blumei Benth as the search model. The overall structure of HDH was similar to that of hydroxyl(phenyl)pyruvate reductase, consisting of two compact domains separated by a deep active cleft. The most significant structural divergence is located around the pocket gate comprising residues A210, T211 and R212, which is located on top of the catalytic triad.
Organizational Affiliation:
State Key Laboratory of Medicinal Chemical Biology, College of Life Sciences, Nankai University, Tianjin, 300071, China, hxdon@126.com.