Substituted benzyloxytricyclic compounds as retinoic acid-related orphan receptor gamma t (ROR gamma t) agonists.
Harikrishnan, L.S., Gill, P., Kamau, M.G., Qin, L.Y., Ruan, Z., O'Malley, D., Huynh, T., Stachura, S., Cavallaro, C.L., Lu, Z., J-W Duan, J., Weigelt, C.A., Sack, J.S., Ruzanov, M., Khan, J., Gururajan, M., Wong, J.J., Huang, Y., Yarde, M., Li, Z., Chen, C., Sun, H., Borowski, V., Murtaza, A., Fink, B.E.(2020) Bioorg Med Chem Lett 30: 127204-127204
- PubMed: 32334911
- DOI: https://doi.org/10.1016/j.bmcl.2020.127204
- Primary Citation of Related Structures:
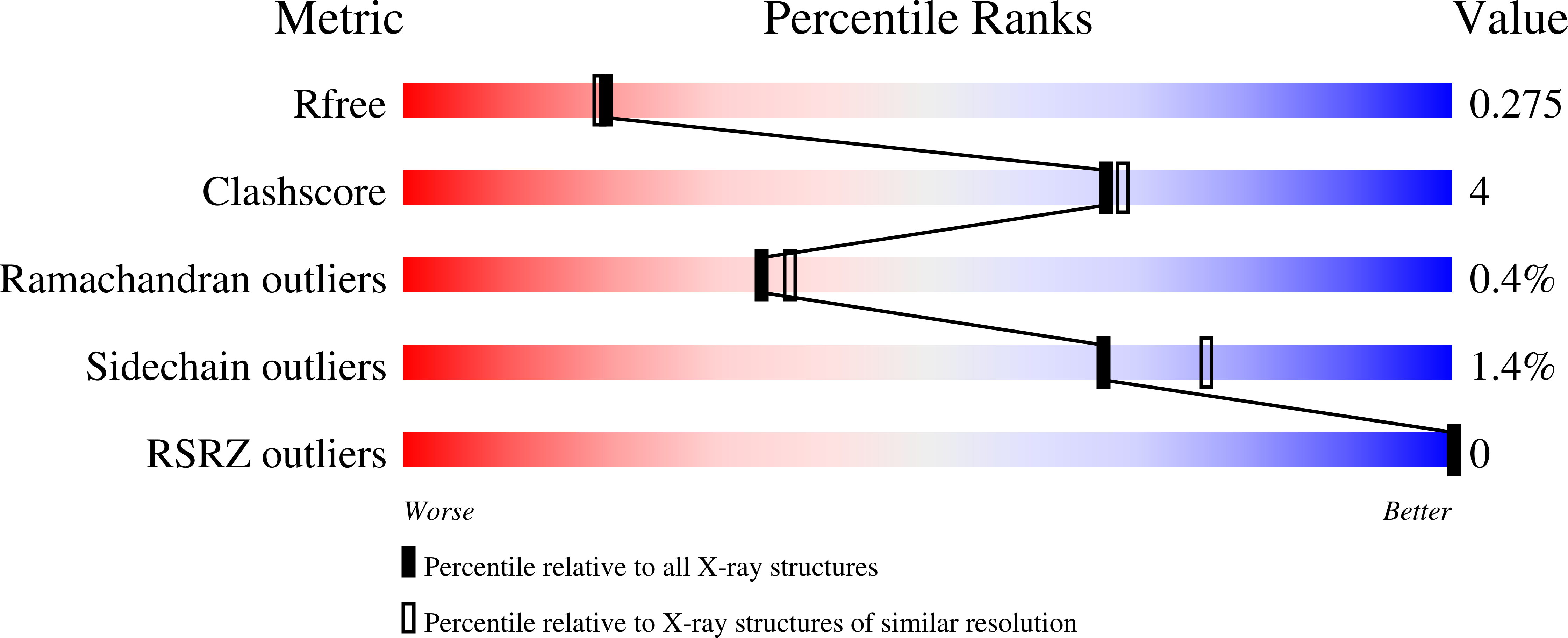
6W9H, 6W9I, 6XAE - PubMed Abstract:
Substituted benzyloxy aryl compound 2 was identified as an RORγt agonist. Structure based drug design efforts resulted in a potent and selective tricyclic compound 19 which, when administered orally in an MC38 mouse tumor model, demonstrated a desired pharmacokinetic profile as well as a dose-dependent pharmacodynamic response. However, no perceptible efficacy was observed in this tumor model at the doses investigated.
Organizational Affiliation:
Department of Chemistry, Bristol Myers Squibb Company, P.O. Box 4000, Princeton, NJ, USA. Electronic address: lalgudi.harikrishnan@bms.com.