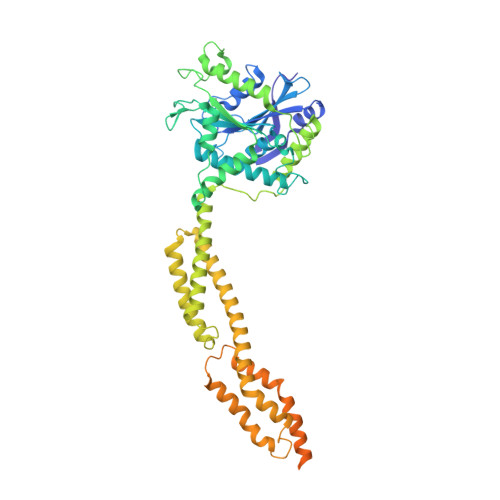
Structural basis of antimicrobial membrane coat assembly by human GBP1.
Kuhm, T., Taisne, C., de Agrela Pinto, C., Gross, L., Giannopoulou, E.A., Huber, S.T., Pardon, E., Steyaert, J., Tans, S.J., Jakobi, A.J.(2025) Nat Struct Mol Biol 32: 172-184
- PubMed: 39394410
- DOI: https://doi.org/10.1038/s41594-024-01400-9
- Primary Citation of Related Structures:
8CQB - PubMed Abstract:
Guanylate-binding proteins (GBPs) are interferon-inducible guanosine triphosphate hydrolases (GTPases) mediating host defense against intracellular pathogens. Their antimicrobial activity hinges on their ability to self-associate and coat pathogen-associated compartments or cytosolic bacteria. Coat formation depends on GTPase activity but how nucleotide binding and hydrolysis prime coat formation remains unclear. Here, we report the cryo-electron microscopy structure of the full-length human GBP1 dimer in its guanine nucleotide-bound state and describe the molecular ultrastructure of the GBP1 coat on liposomes and bacterial lipopolysaccharide membranes. Conformational changes of the middle and GTPase effector domains expose the isoprenylated C terminus for membrane association. The α-helical middle domains form a parallel, crossover arrangement essential for coat formation and position the extended effector domain for intercalation into the lipopolysaccharide layer of gram-negative membranes. Nucleotide binding and hydrolysis create oligomeric scaffolds with contractile abilities that promote membrane extrusion and fragmentation. Our data offer a structural and mechanistic framework for understanding GBP1 effector functions in intracellular immunity.
- Department of Bionanoscience, Kavli Insitute of Nanoscience, Delft University of Technology, Delft, The Netherlands.
Organizational Affiliation: