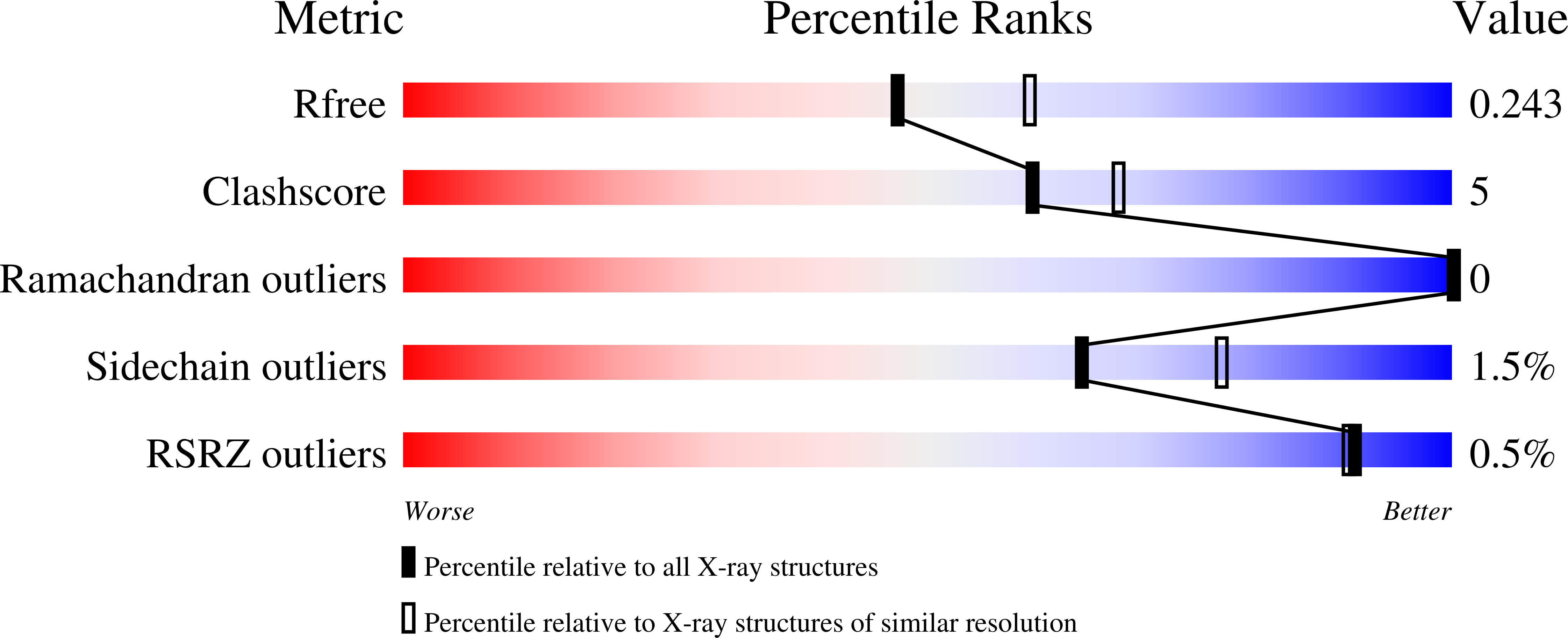
Crystal structures and catalytic mechanism of the C-methyltransferase Coq5 provide insights into a key step of the yeast coenzyme Q synthesis pathway.
Dai, Y.N., Zhou, K., Cao, D.D., Jiang, Y.L., Meng, F., Chi, C.B., Ren, Y.M., Chen, Y., Zhou, C.Z.(2014) Acta Crystallogr D Biol Crystallogr 70: 2085-2092
- PubMed: 25084328
- DOI: https://doi.org/10.1107/S1399004714011559
- Primary Citation of Related Structures:
4OBW, 4OBX - PubMed Abstract:
Saccharomyces cerevisiae Coq5 is an S-adenosyl methionine (SAM)-dependent methyltransferase (SAM-MTase) that catalyzes the only C-methylation step in the coenzyme Q (CoQ) biosynthesis pathway, in which 2-methoxy-6-polyprenyl-1,4-benzoquinone (DDMQH2) is converted to 2-methoxy-5-methyl-6-polyprenyl-1,4-benzoquinone (DMQH2). Crystal structures of Coq5 were determined in the apo form (Coq5-apo) at 2.2 Å resolution and in the SAM-bound form (Coq5-SAM) at 2.4 Å resolution, representing the first pair of structures for the yeast CoQ biosynthetic enzymes. Coq5 displays a typical class I SAM-MTase structure with two minor variations beyond the core domain, both of which are considered to participate in dimerization and/or substrate recognition. Slight conformational changes at the active-site pocket were observed upon binding of SAM. Structure-based computational simulation using an analogue of DDMQH2 enabled us to identify the binding pocket and entrance tunnel of the substrate. Multiple-sequence alignment showed that the residues contributing to the dimeric interface and the SAM- and DDMQH2-binding sites are highly conserved in Coq5 and homologues from diverse species. A putative catalytic mechanism of Coq5 was proposed in which Arg201 acts as a general base to initiate catalysis with the help of a water molecule.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at the Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui 230027, People's Republic of China.