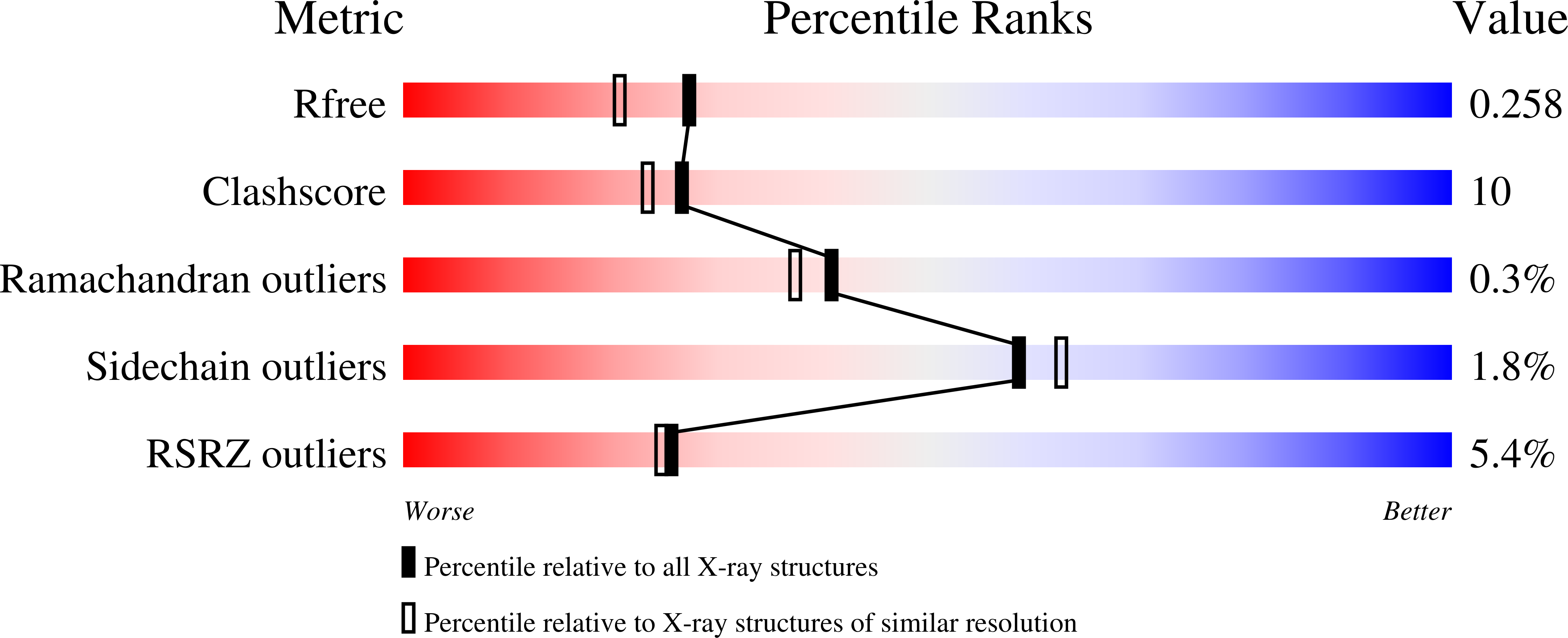
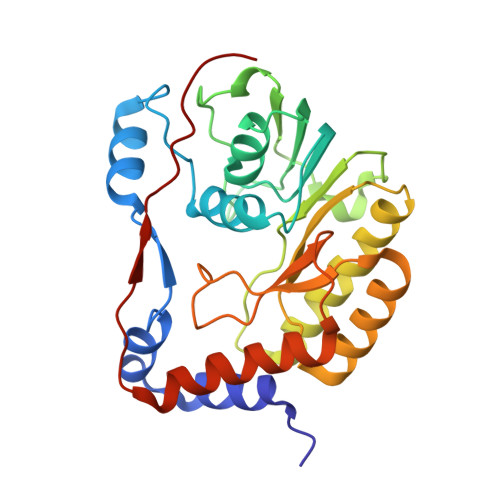
The structure of the binary methyltransferase-SAH complex from Zika virus reveals a novel conformation for the mechanism of mRNA capping.
Chatrin, C., Talapatra, S.K., Canard, B., Kozielski, F.(2018) Oncotarget 9: 3160-3171
- PubMed: 29423037
- DOI: https://doi.org/10.18632/oncotarget.23223
- Primary Citation of Related Structures:
5NJU, 5NJV - PubMed Abstract:
Zika virus, a flavivirus like Dengue and West Nile viruses, poses a significant risk as a pathogen in the category of emerging infectious diseases. Zika infections typically cause nonspecific, mild symptoms, but can also manifest as a neurological disorder like Guillain-Barré syndrome. Infection in pregnant women is linked to microcephaly in newborn infants. The methyltransferase domain of the non-structural protein 5 is responsible for two sequential methylations of the 5'-RNA cap. This is crucial for genome stability, efficient translation, and escape from the host immune response. Here we present the crystal structures of the Zika methyltransferase domain in complex with the methyl-donor SAM and its by-product SAH. The methyltransferase-SAH binary complex presents a new conformation of a "closed" or "obstructed" state that would restrict the binding of new RNA for capping. The combination and comparison of our new structures with recently published Zika methyltransferase structures provide a first glimpse into the structural mechanism of Zika virus mRNA capping.
Organizational Affiliation:
Department of Pharmaceutical and Biological Chemistry, UCL School of Pharmacy, WC1N 1AX, London, United Kingdom.