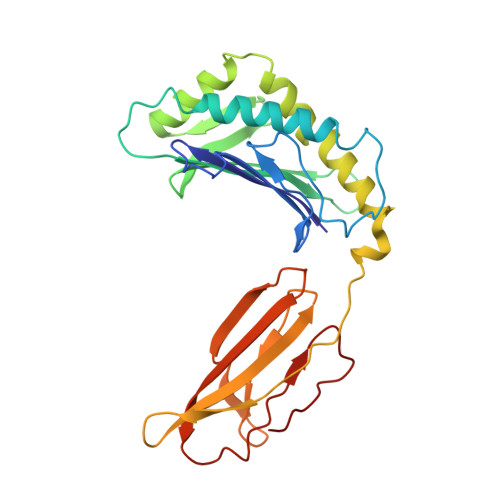
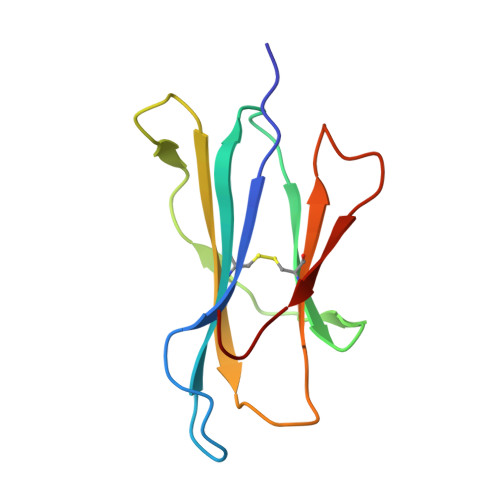
Human Neonatal Fc Receptor Is the Cellular Uncoating Receptor for Enterovirus B.
Zhao, X., Zhang, G., Liu, S., Chen, X., Peng, R., Dai, L., Qu, X., Li, S., Song, H., Gao, Z., Yuan, P., Liu, Z., Li, C., Shang, Z., Li, Y., Zhang, M., Qi, J., Wang, H., Du, N., Wu, Y., Bi, Y., Gao, S., Shi, Y., Yan, J., Zhang, Y., Xie, Z., Wei, W., Gao, G.F.(2019) Cell 177: 1553-1565.e16
- PubMed: 31104841
- DOI: https://doi.org/10.1016/j.cell.2019.04.035
- Primary Citation of Related Structures:
6ILJ, 6ILK, 6ILL, 6ILM, 6ILN, 6ILO, 6ILP - PubMed Abstract:
Enterovirus B (EV-B), a major proportion of the genus Enterovirus in the family Picornaviridae, is the causative agent of severe human infectious diseases. Although cellular receptors for coxsackievirus B in EV-B have been identified, receptors mediating virus entry, especially the uncoating process of echovirus and other EV-B remain obscure. Here, we found that human neonatal Fc receptor (FcRn) is the uncoating receptor for major EV-B. FcRn binds to the virus particles in the "canyon" through its FCGRT subunit. By obtaining multiple cryo-electron microscopy structures at different stages of virus entry at atomic or near-atomic resolution, we deciphered the underlying mechanisms of enterovirus attachment and uncoating. These structures revealed that different from the attachment receptor CD55, binding of FcRn to the virions induces efficient release of "pocket factor" under acidic conditions and initiates the conformational changes in viral particle, providing a structural basis for understanding the mechanisms of enterovirus entry.
Organizational Affiliation:
CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, 100101 Beijing, China; CAS Center for Influenza Research and Early-Warning (CASCIRE), Chinese Academy of Sciences, 100101 Beijing, China.