Rational Design and Synthesis of Selective PRMT4 Inhibitors: A New Chemotype for Development of Cancer Therapeutics*.
Sutherland, M., Li, A., Kaghad, A., Panagopoulos, D., Li, F., Szewczyk, M., Smil, D., Scholten, C., Bouche, L., Stellfeld, T., Arrowsmith, C.H., Barsyte, D., Vedadi, M., Hartung, I.V., Steuber, H., Britton, R., Santhakumar, V.(2021) ChemMedChem 16: 1116-1125
- PubMed: 33513288
- DOI: https://doi.org/10.1002/cmdc.202100018
- Primary Citation of Related Structures:
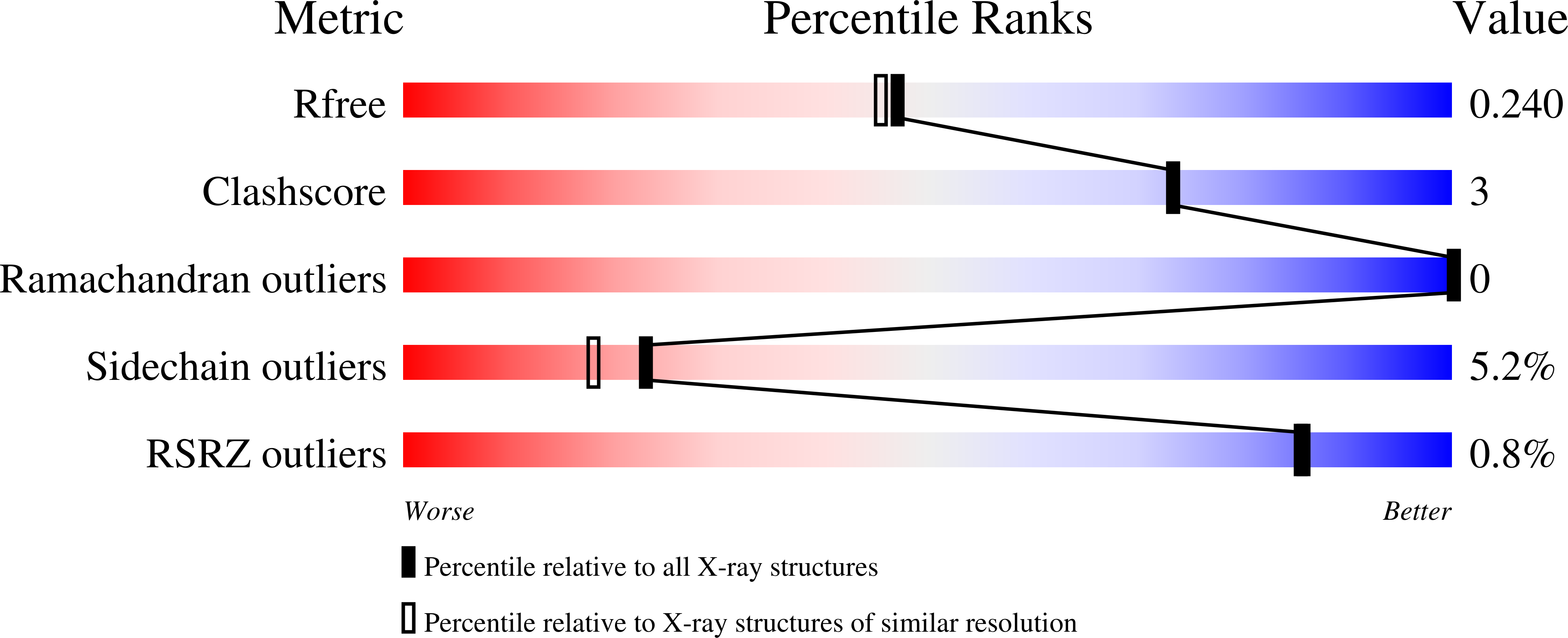
7NR4 - PubMed Abstract:
Protein arginine N-methyl transferase 4 (PRMT4) asymmetrically dimethylates the arginine residues of histone H3 and nonhistone proteins. The overexpression of PRMT4 in several cancers has stimulated interest in the discovery of inhibitors as biological tools and, potentially, therapeutics. Although several PRMT4 inhibitors have been reported, most display poor selectivity against other members of the PRMT family of methyl transferases. Herein, we report the structure-based design of a new class of alanine-containing 3-arylindoles as potent and selective PRMT4 inhibitors, and describe key structure-activity relationships for this class of compounds.
Organizational Affiliation:
Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC V5A 1S6, Canada.